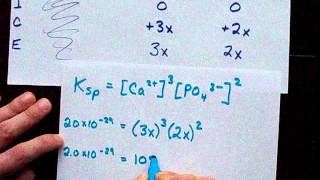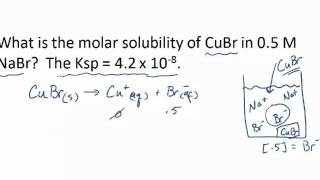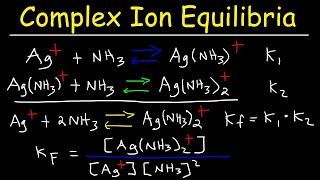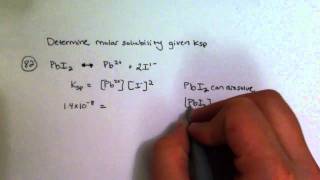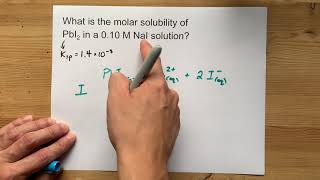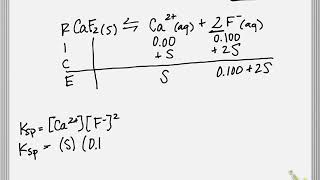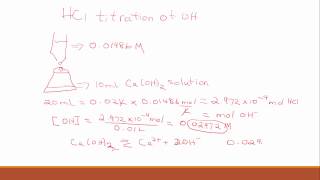Solubility Equilibrium Practice Problems |

|
|
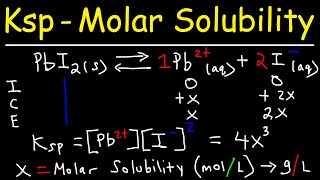
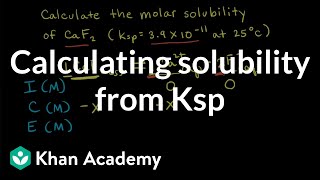
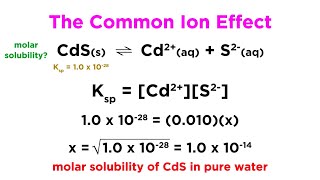
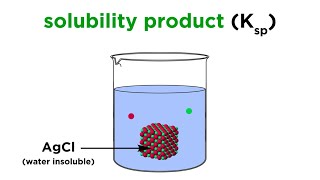
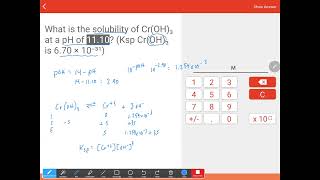
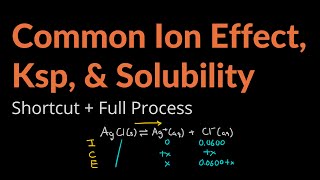
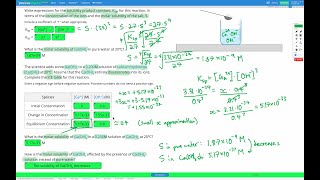
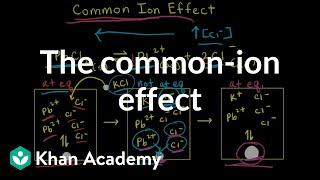
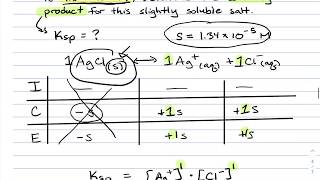
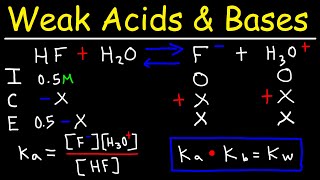
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali.
Access more videos on Chemical Equilibrium Simplified including past tutorial sheets by registering with us.. I have many more videos on Equilibrium and Chemistry including Kinetics, ThermoChemistry, ElectroChemistry and several practice questions from past papers. More videos in Chemistry including tutorial sheet solutions, link below to register. https://learn.transcendedinstitute.com/ Upon registration you get to access all other topics from Stoichiometry till Organic chemistry Enrol for Chemistry now and Access(Link) https://learn.transcendedinstitute.com/ Transcended Institute offers online lessons to students studying Natural Sciences, A-level students and High school students doing MATHEMATICS, CHEMISTRY, PHYSICS AND BIOLOGY. Do you wish to register? don't hesitate, get in touch with us on whatsapp using the link below. https://wa.me/message/474U6ZWKDJ2JC1 Alternatively Call/whatsapp 260767729927. You can also e-mail us at transcendedinstitute@gmail.com Channel link: https://www.youtube.com/c/TRANSCENDED... VISIT OUR FACEBOOK PAGE https://www.facebook.com/TRASCENDED VISIT OUR WEBSITE https://transcendedinstitute.com/ REGISTER FOR OUR CLASSES AND UP YOUR GRADES. #LearnTheSmartWay |