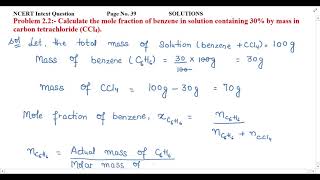
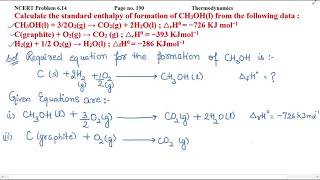
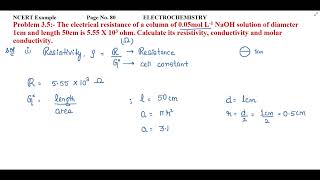
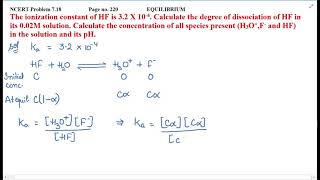
Calculate the molar mass of Acetic acid (CH3COOH)? |

|
|
Calculate the molar mass of Acetic acid (CH3COOH)?
|

Determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% dioxygen by mass.

34.05mL of phosphorous vapor weigh 0.0625g at 546oC &1bar pressure What is molar mass of phosphorous

Pay load is defined as the difference between the mass of displaced air and the mass of the balloon.