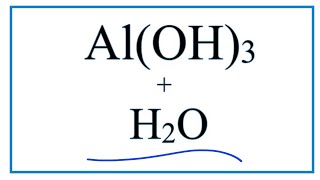Equation for Al(OH)3 + H2O (Aluminum Hydroxide + Water) |

|
|
In this video we will describe the equation Al(OH)3 + H2O and write what happens when Al(OH)3 is dissolved in water.
We might expect that when Al(OH)3 is dissolved in H2O (water) it would dissociate (dissolve) into Al 3+ and OH- ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water. We can consult a solubility table to check and determine if Al(OH)3 will dissolve in water. There are also a set of general rules to help remember which compounds are soluble, insoluble, or partially soluble. When we check the solubility table we see that Al(OH)3 is insoluble. Essentially you end up with wet Al(OH)3 when you mix Al(OH)3 with water. If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations: Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88Fs More Practice Balancing: https://youtu.be/Qci7hiBy7EQ Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). Image from: https://en.wikipedia.org/wiki/Aluminium_hydroxide#/media/File:Hydroxid_hlinit%C3%BD.PNG |