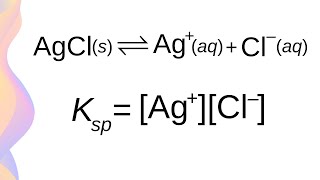Common Ion Effect // HSC Chemistry |

|
|
The Common Ion Effect is a phenomenon in solution equilibria as part of HSC Chemistry's Module 5: Equilibrium and Acid Reactions. Calculations involving the common ion effect is included.
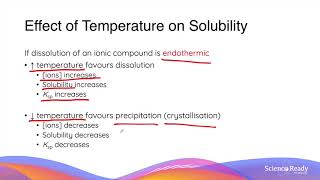
Syllabus Dotpoints * conduct an investigation to determine solubility rules, and predict and analyse the composition of substances when two ionic solutions are mixed, for example: – potassium chloride and silver nitrate – potassium iodide and lead nitrate – sodium sulfate and barium nitrate (ACSCH065) * derive equilibrium expressions for saturated solutions in terms of Ksp and calculate the solubility of an ionic substance from its Ksp value * predict the formation of a precipitate given the standard reference values for Ksp What is the Common Ion Effect? What happens in common ion effect? Common ion effect calculations. Visit our website: http://www.scienceready.com.au Follow our Instagram page: http://www.instagram.com/hscscienceready Like our Facebook page: http://www.facebook.com/hscscienceready |