Diphtheria Toxin Action Mechanism | Signalling Pathway |

|
|
Diphtheria toxin is an exotoxin secreted by Corynebacterium, the pathogenic bacterium that causes diphtheria.
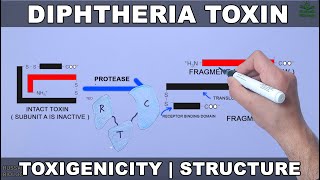
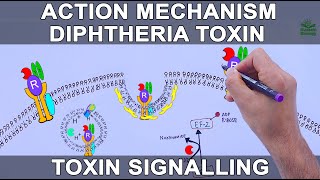
Diphtheria toxin is composed of two subunits bound by a disulfide bond between cysteine residues: the amino-terminal A-subunit contains the catalytic (C) domain (not shown), and the carboxyl-terminal B-subunit contains a membrane-inserting translocation (T) domain and a receptor-binding (R) domain (not shown). Diphtheria toxin secreted from the bacterial cell binds to the proheparin-binding epidermal growth factor-like growth factor (HB-EGF), which acts as a DT receptor. DT enters the cell via receptor-mediated endocytosis through clathrin-coated pits: the R domain recognizes the HB-EGF receptor on human epithelial cells, leading to endocytosis of the entire receptor–toxin complex. Endosome-associated proteases partially cleave the bond between the DT subunits, and exposure of the DT to the acidic conditions triggers a conformational change that enables the T domain to insert into the endosome membrane and the subsequent translocation of the A-subunit across the endosomal membrane into the cytosol. The T domain is thought to be primarily responsible for membrane insertion, although the C and R domains have also been shown to be associated with membranes262. In the cytosol, the C domain catalyses the transfer of the ADP-ribose moiety of nicotinamide adenine dinucleotide (NAD) onto the elongation factor 2 (EF-2). EF-2 is a member of the GTP-binding translation elongation factor family. This protein is an essential factor for the cell protein synthesis and enables the transfer of the peptidyl tRNA–mRNA complex from the ribosome to the peptidyl site during protein synthesis. DT targets a post-transcriptionally modified histidine diphthamide on EF-2 (ref.263). ADP-ribosylation of this unique diphthamide residue prevents EF-2 translocation activity, resulting in inhibition of protein production. |