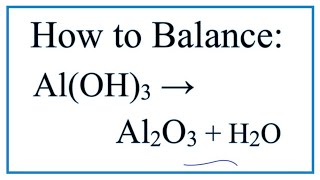
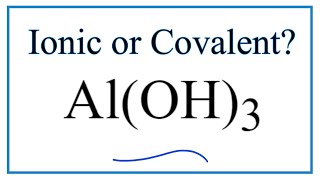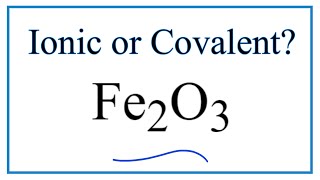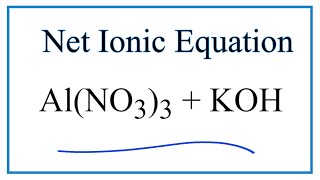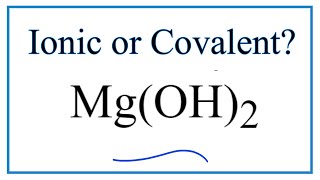Is Al(OH)3 (Aluminum hydroxide) Ionic or Covalent? |

|
|
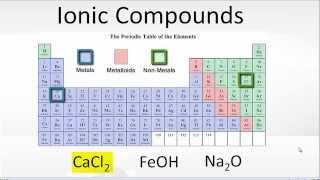
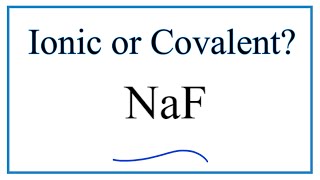
To tell if Al(OH)3 (Aluminum hydroxide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Al is a metal and OH is a group of non-metals. Here the group of nonmetals make up a polyatomic ion. When we have a metal and a group of non-metals the compound is usually considered ionic.
Because we have a metal and non-metals in Al(OH)3 there will be a difference in electronegativity between the metal and group of nonmetals. This difference results in an electron(s) being transferred from the metal (lower electronegativity) to the non-metal (higher electronegativity). The results in the metal becoming a positive ion and the group of non-metals a negative polyatomic ion. The two opposite charges are attracted and form the ionic bond between ions in Aluminum hydroxide. --- Helpful Resources Metals, Non-Metals on the P- Table: https://youtu.be/OoooStZQHdA Ionic, Covalent, & Polar Covalent: https://youtu.be/OHFGXfWB_r4 Electronegativity for each element: https://en.wikipedia.org/wiki/Electronegativity Memorizing Polyatomic Ions: https://youtu.be/vepxhM_bZqk Finding Ionic Charge: https://youtu.be/N4N1Njh7nCo --- Because we have a combination of a metal and non-metal Al(OH)3 (Aluminum hydroxide) is considered an ionic compound. In general, ionic compounds: - form crystals. - have high melting points and boiling points. - are hard and brittle. - conduct electricity when dissolved in water. - as solids do not conduct electricity. For more chemistry help, see http://www.Breslyn.org. |