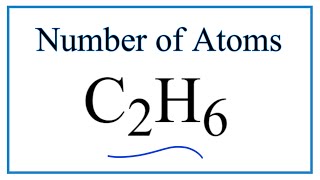
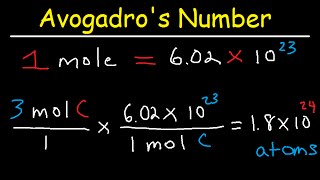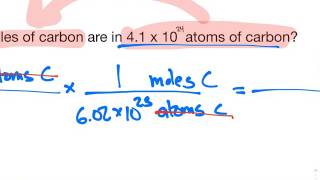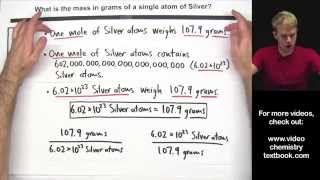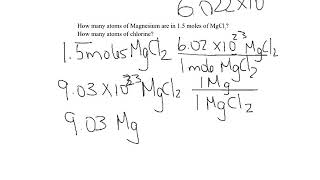Number of Atoms in a Mole |

|
|
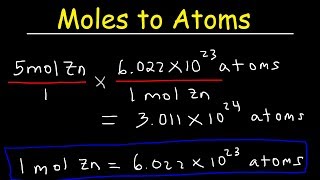
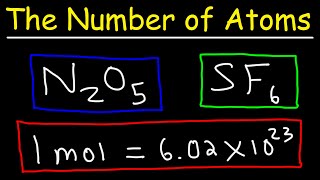
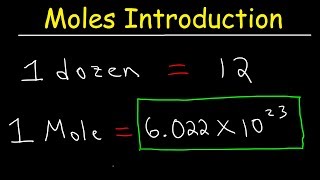
In this video well learn to how to determine the number of atoms in one mole of a substance. Simply put, one mole of a substance (atoms, molecules, ions, etc.) will be Avogadro's Number (6.02 x 10^23).
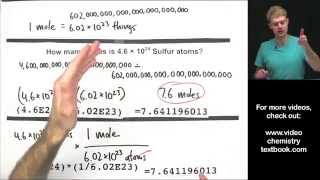
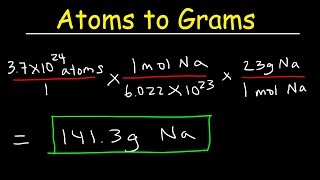
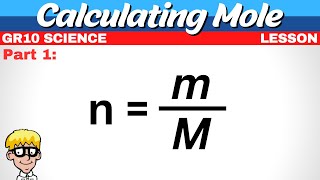
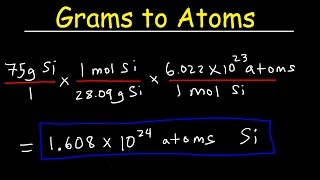
We can also determine the number of a specific type of atom in one mole of a molecule. In the video we find the number of Hydrogen (H) atoms in one mole of CH4 (methane). Often the term "particles" will be used to describe atoms, molecules, ions, etc. Converting from moles to particles we multiple by 6.02 x 10^23. If we're given the number of particles we can convert to moles by dividing by Avogadro's Number (6.02 x 10^23). For more help with mole conversions and more: • Understanding the Mole: https://youtu.be/DyLktMPTuHY • More Moles to Grams Practice: https://youtu.be/aIv5nr8ZNyw • Molar Mass in Three Easy Steps: https://youtu.be/o3MMBO8WxjY • Moles - Gram Conversions: https://youtu.be/aIv5nr8ZNyw • How to Balance Chemical Equations: https://youtu.be/zmdxMlb88Fs • Mole Ratio: https://youtu.be/i71BMVlrMiw • Reaction Stoichiometry: https://youtu.be/rrTqOsZPpaU My chemistry website: http://www.Breslyn.org Note: converting between moles and grams is the cornerstone of being successful in stoichiometry, the study of chemical quantities. Take the time to learn mole conversions and you will find chemistry is much easier. The use of conversion factors (also called factor-label method or dimensional analysis) is a more general technique for converting quantities. Once you understand how it works it can be applied to many different conversion (as long as you know the conversion factor). |