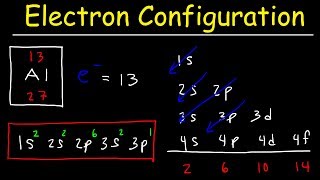
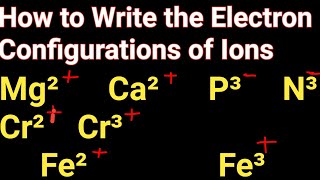
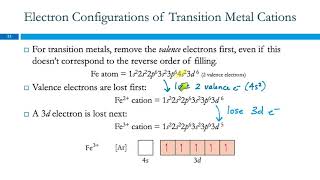
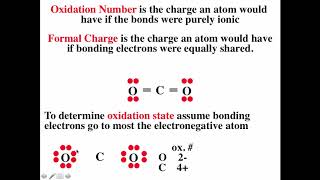
How to Write the Electron Configuration for Ions |

|
|
When we write the electron configuration for ions, we need to understand that ions will have a different number of electrons than the neutral element in the ground state.
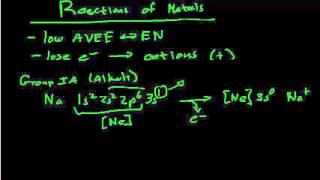
When an atom loses electrons it becomes a positive ion (called a cation). Because cations have lost an electron(s) the configuration will have fewer electrons. For example, neutral Na has the configuration of: 1s2 2s2 2p6 3s1 When it forms the Na+ ion it loses the electron in the 3s1 and the electron configuration becomes: 1s2 2s2 2p6 which is the same configuration as the Noble Gas Neon (Ne). For neutral Cl, the electron configuration is: 1s2 2s2 2p6 3s2 3p5 To become Cl-, the Chloride ion, it gains an electron to have a configuration of 1s2 2s2 2p6 3s2 3p6 which is the same electronic configuration as the Noble Gas Argon (Ar) A frequent question in writing the electron configuration for ions and ionic compounds is where the electron went or (or came from). In the examples above the Na could lose an electron to Cl and the result would be Na+ and Cl- which would then be attracted to for the ionic compound NaCl. |