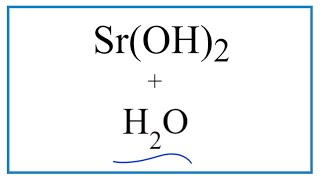
Equation for Sr(OH)2 + H2O (Strontium hydroxide + Water) |

|
|
In this video we will describe the equation Sr(OH)2 + H2O and write what happens when Sr(OH)2 is dissolved in water.
When Sr(OH)2 is dissolved in H2O (water) it will dissociate (dissolve) into Sr 2+ and OH - ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water. The equation for Sr(OH)2 ( Strontium hydroxide and H2O sometimes isn’t considered a chemical reaction since it is easy to change the Sr 2+ and OH - back to Sr(OH)2 (just let the H2O evaporate). At the same time, the Sr(OH)2 is a very different substance than Sr 2+ and OH -. If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations: Balancing Equations in 5 Easy Steps: https://youtu.be/zmdxMlb88Fs More Practice Balancing: https://youtu.be/Qci7hiBy7EQ Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo). |